Concentration Of Naoh In Titration
The technique known as titration is an belittling method commonly used in chemistry laboratories for determining the quantity or concentration of a substance in a solution. In a titration, an analyte -- the substance whose quantity or concentration is to exist determined -- is reacted with a advisedly controlled volume of solution of accurately-known concentration called a standard solution. The standard solution (also known as the titrant) is unremarkably added to the solution containing the analyte by means of a buret, a piece of volumetric glassware capable of accurately measuring solution volumes.
There are many types of titrations in common apply in the analytical chemistry laboratory. Each blazon uses a different kind of chemic reaction. Examples of titration types include
The about commonly studied blazon is the acid-base titration. For this project we focus our attention exclusively on this type, and we develop a mathematical model that describes the relationship between the volume of added titrant (a base) and changes in concentration of the analyte (an acrid). The model presented would demand but slight modifications to be applicable to other titration types listed above.
In a typical acid-base titration experiment, the solution containing the analyte (an acrid of unknown identity and/or concentration) is placed into a container, and the titrant (a base of accurately-known concentration) is slowly added from the buret in small increments (see Effigy 1).
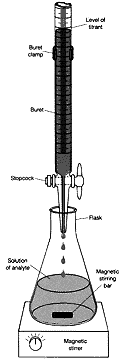
Effigy 1.
After the addition of each increment of base, the volume of base is carefully read from the buret (measured to the nearest hundredth of a milliliter) and the hydrogen ion (H+) concentration of the solution is measured with a pH meter. The pH meter gives the hydrogen ion concentration in terms of pH, which is simply the negative logarithm (common log, i.e., base ten) of the hydrogen ion concentration:
where [H+] represents the concentration of H+.
The hydrogen ion concentration is directly related to the amount of acid present in the solution at any item pace in the titration according to the post-obit chemical reaction:
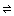 H+ hydrogen ion + A- (anion of the acrid)
H+ hydrogen ion + A- (anion of the acrid) The measurement of the hydrogen ion concentration (or pH) at each point in the titration allows us to find the location of the equivalence betoken, that book of base which reacts completely with the unknown concentration of acid. It is at this equivalence point that the amount of base added is chemically equal to the amount of acid present. By chemically equal we mean that the number of molecules of base added is just plenty to completely react with all of the molecules of acid originally present -- so that all of the acid molecules are, in a sense, used up. By knowing the book of base at the equivalence point, as well as the concentration of the base of operations, one can calculate (among other things) the initial concentration of the acrid.

Figure 2. This is a typical titration graph plotting volume of titrant added versus pH of solution. Notice that just a small change in pH occurs equally nearly of the titrant is added, then near the terminate of the titration in that location is a sudden, rapid modify in pH. The sudden change occurs at the equivalence point. The location of the equivalence signal is, therefore, adamant past identifying the point of fastest increment.
The chemic reaction that occurs between the acrid and the base allows 1 to calculate the initial concentration (or amount) of the acrid. For example, in the titration of hydrochloric acid (HCl) with a base such as sodium hydroxide (NaOH), the chemical reaction between these two species would have to exist known. The reaction is as follows:
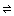 NaCL + Htwo0 NaCL + Htwo0 | (1) |
This reaction states that one molecule of HCl volition react with ane molecule of NaOH to produce 1 molecule of the salt, sodium chloride (NaCl), and ane molecule of water. The chemical equation allows us to summate the concentration of a solution of HCl by titration with the base NaOH (where the concentration of NaOH is accurately known).
Allow's suppose that our solution is 0.02500 Fifty of an unknown concentration of the acid, HCl. Nosotros wish to find its concentration by titration with 0.1000 One thousand NaOH. (Thou is the notation for the concentration unit called molarity, which is defined equally the number of moles of a substance per liter of solution. A mole is equal to six.022 10 1023 molecules.) By doing the titration and making a plot of the volume of NaOH added versus the resulting pH of the solution, we observe that the equivalence indicate occurs at 0.04398 L of NaOH. (This is the point where the plot appears to increase most rapidly.) Recall that at the equivalence betoken the amount of base added is chemically equal to the amount of acrid present in the solution. Co-ordinate to the chemic reaction (1) between HCl and NaOH, 1 molecule of HCl will react with exactly 1 molecule of NaOH. Therefore, the number of moles of base of operations needed to react with all of the acid nowadays is the same as the number of moles of acid present in the solution. So, at equivalence signal,
We can calculate the number of moles of NaOH at the equivalence point by multiplying its concentration (in terms of molarity or moles/L) by the volume of NaOH added in club to attain the equivalence point:
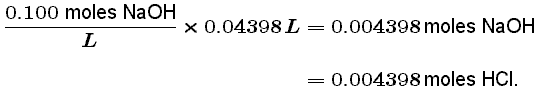
This tells the states that there were 0.004398 moles of HCl in our original 0.02500 L solution. Finally, we can calculate the unknown concentration of HCl:
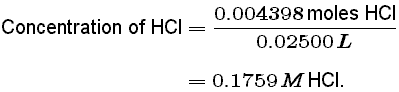
Concentration Of Naoh In Titration,
Source: https://services.math.duke.edu/education/ccp/materials/calculus_projects/TitrationProj/Titration1.html
Posted by: samonsatrom1955.blogspot.com


0 Response to "Concentration Of Naoh In Titration"
Post a Comment